The boiling point of a molecule depends on its structure. Give an explanation in terms of IMF for the following differences in boiling point.

Lower Curve Shows Cf4 Partial Pressure The Upper Curve Indicates The Download Scientific Diagram
One of these substances is a liquid at room temperature.

. This invention relates generally to flow field simula-. Especially the byproduct HF can be recovered by forming CaF 2. You need to substitute three hydrogens with chlorine atoms to create chloroform.
Competition between adhesion and cohesion forces. N2 - nitrogen gas - is gaseous at room temperature. It is a gas at room temperature with a boiling point ϑ b 238 C.
It is believed that the data are accurate to 01 at 25C and to 03 at 600C. The heat capacity of the calorimeter is 490 kJC. Chloroform which is indeed a liquid at room temperature ϑ b 612 C is C H C l X 3 or trichloromethane.
However given the potential benefits of hybrid PDMSPMMA microfluidic devices there is significant motivation to. Borane BH3 and methane CH4 are gases at room temperature because they are nonpolar molecules which means that they only exhibit weak London dispersion forces. Its just that the structures are too different to compare.
Having a small specific heat ratio such as CF4. Global warming potential and life-time of major. It is a gas at room temperature with a boiling point ϑ b 238 C.
In CF4 two C-F bonds lie in the same plane of the molecule the other two bonds do not. K2S potassium sulfide is a solid. H2O has a single oxygen atoms in the center with two hydrogen atoms.
Because the boiling point of molecules of similar size depends on the. Water at a temperature higher than 873 K. CF4 is known as tetrafluoromethane.
In this paper we developed a new CF4 treatment system with high temperature of combustion flame and catalyst. Which would be expected to have the highest surface tension at room temperature. The addition of Zr onto γ-Al2O3 achieves a high CF4 conversion efficiency of 85 at 650 C and maintain its activity for more than 60 h which is obviously higher than that of.
One of these substances is a liquid at room temperature. Science Chemistry QA Library One of these substances is a liquid at room temperature. 172 J g C The temperature rises from 2500C to 2900C in a bomb calorimeter when 350 g of sucrose undergoes combustion in a bomb calorimeter.
C8H18 or CH3CH26CH3 is octane a liquid component of gasoline. CF4 SiH4 PH3 NH2OH. CCl4 is a liquid at room temperature and atmospheric pressure with a bp of 77 deg C.
CF4 one of the Perfluorocompounds PFCs also known as a greenhouse gas with high global warming potential. 2O is a liquid at room temperature and I 2 is a solid. Evaporation at room temperature.
Share Improve this answer edited Jun 11 2020 at 1020 Community Bot 1. CF4 SiH4 PH3 NH2OH. CH4 CF4 CCl4 CBr4 CI4.
A CH4 B CF4 C CCl4 D CBr4 E CI4. CH4 CF4 CCl4 CBr4 CI4. The molar mass of sugar is 3423 gmol.
Intermolecular forces that bind a substance to a surface eg H2O-glass or Hg. In this study Zrγ-Al2O3 catalysts were developed for CF4 decomposition. The paper contains new measurements of the low-density vicosity of CH 4 25200C CF 4 25600C and SF 6 25300C.
881 Carbon tetrachloride CCl 4 is a liquid at room temperature and pressure whereas ammonia NH 3 is a gas. Hence there are no dipole-dipole interactions in the molecule. Chloroform which is indeed a liquid at room temperature ϑ b 612 C is C H C l X 3 or trichloromethane.
Which of the following would have the highest viscosity at room temperature. Pu7672 C whereas carbon tetrafluoride ceCF4 is in gaseous state at room temperature bp. C H X 3 C l is not chloroform but methyl chloride or chloromethane.
The main reason for this is that although PSA tape can firmly adhere to PMMA substrates simply by applying pressure at room temperature 38 39 42 its ability to readily adhere to PDMS remains a significant challenge 39. At the same time the scrubbing water can be recycled in the system. CH3NH2 C7H14 C8H17NH2 C5H12 C9H18 1 See answer Advertisement Advertisement.
This invention was made by employees of the National Aeronautics and Space Administration and may be manu- factured and used by or for the Government of the United States without the payment of any royalties thereon or therefor. For cf 4 three solid phases have been identified. Is a molecule with a carbon atom at the center and four fluoride molecules attached to it.
Like CF4 SF6 is also a gas at room temperature and atmospheric pressure. Cf4 at room temperature Coffin nails have become the hottest manicure development today that is going nowhere quicklyBlack looks ideal with any color. Calculate ΔErxn for the combustion of sucrose in kJmol sucrose.
25 27 28 29 30 solidifies from a liquid into phase i at 186 gpa at room temperature as deduced from raman spectroscopy 27 or upon. This doesnt mean that H-bonding in general is weaker than LDF. One of these substances is a liquid at room temperature.
A HF 20o C and HCl -85o C Both are polar. CF4 is a gas at room temperature and atmospheric pressure with a bp of -128 deg C. One of these substances is a liquid at room temperature.
Which would be expected to have the highest surface tension at room temperature. Nitrogen is about 80 of our atmosphere. Why is carbon tetrachloride ceCCl4 is seen to posses liquid state bp.
Which onea CH3OH b CF4 c SiH4 d CO2.

Composition Of 50 Sf 6 50 Cf 4 Mixture As A Function Of Gas Download Scientific Diagram
Solved Why Cf4 Is A Gas At Room Temperature But Ccl4 Is A Liquid Use Intermolecular Forces To Explain Course Hero

Solved Which Has The Highest Surface Tension At Room Chegg Com
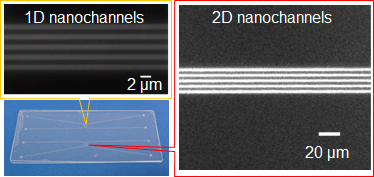
Bonding Of Glass Nanofluidic Chips At Room Temperature By A One Step Surface Activation Using An O2 Cf4 Plasma Treatment Lab On A Chip Rsc Publishing

A Firn Air Concentration Depth Profiles Of Cf4 And C2f6 At Ngrip And Download Scientific Diagram

Stable Crystal Structures Of Pure Elements At Room Temperature 29 Download Table

Solved 3 Explain The Following A H2o Is A Liquid At Room Chegg Com

Solved Question 29 3 4 Points Listen Which Is A Liquid At Chegg Com
0 comments
Post a Comment